Navigating the Latest Changes in In Vitro Micronucleus Testing and What It Means for You
Key Takeaways
1. Spotlight on Test Guideline 487 Updates: Uncover key adjustments introduced in the updated guidelines and their significance in genotoxicity testing.
2. Understanding the Impact: Learn how these revisions influence Inotiv’s testing methodologies and what it means for your genotoxicity assessments.
As the field of chemical safety assessment continues to evolve, so do the guidelines that sustain it. On July 4, 2023, the OECD Test Guideline 487, a cornerstone of genotoxicity testing, underwent subtle revisions. In this blog, we examine these minor adjustments, share our approach to their integration, and clarify how they may impact your genotoxicity testing experience.
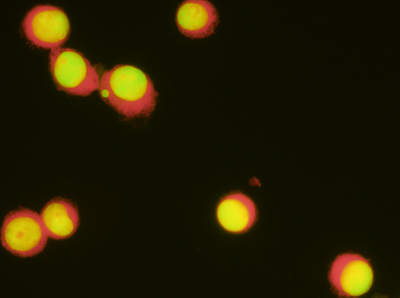
Micronucleus is a small membrane-bounded compartment with DNA content encapsulated by a nuclear envelope and spatially separated from the primary nucleus.
Understanding OECD Test Guideline 487 The OECD 487 guideline, focusing on the in vitro mammalian cell micronucleus test, has long been vital in identifying potential genotoxic effects. Studies performed under Test Guideline 487 are used to detect micronuclei within the cytoplasm of interphase cells that can arise from acentric chromosome fragments or whole chromosomes that fail to migrate during the anaphase stage of cell division. While the core principles of the test remain unchanged, several subtle updates have been introduced to enhance clarity and precision.
Key Updates and Implications Specific alterations have been made to the guideline in response to scientific progress and evolving practices. These updates include:
• Removal of the term “cytostasis” in designated sections
• A refined cytotoxicity requirement
• Redefined parameters in Annex 1 and 2
While seemingly minor, these adjustments aim to improve the consistency and accuracy of genotoxicity assessments.
Our Approach to Change
We’re committed to providing reliable and high-quality testing. The integration of these revised guidelines into our existing protocols showcases our dedication to staying current with best practices. Clients can rest assured that our rigorous testing protocols conform to the latest guidelines, guaranteeing the same consistent and accurate results you’ve come to expect from us.
What OECD 487 Updates Mean for You
The refined cytotoxicity requirement and redefined parameters contribute to a more comprehensive evaluation of potential hazards. As a result, clients can make more informed decisions about the safety of their products, guided by robust and reliable data. Importantly, these updates have minimal impact on our existing procedures, ensuring a smooth transition without altering calculations or methodologies.
Conclusion
While the recent updates to OECD Test Guideline 487 may appear subtle, their significance should not be underestimated. We acknowledge and embrace these changes, recognizing their role in continually improving the quality of genotoxicity testing. As our valued client, you can trust that our unwavering dedication to excellence ensures a seamless transition and sustained reliability in your genotoxicity assessments.
Learn more about our genetic toxicology testing services, including in vitro micronucleus, by clicking here.
Reference Link To Revised OECD 487 Guideline: OECD 487 Guideline


